‘Aqueous film-forming foam’ or AFFF is a foamy white substance. Which is very effective in suppressing fire from liquid. This foam works great in extinguishing jet fuel fires. The US military and several other organizations have been using this foam for decades.
However, whenever AFFF foam is used in a firefighting practice or in an actual fire, another problem arises. The solution of which is quite difficult. Because once this foam is sprayed, it can’t be safely collected or destroyed—plus it’s toxic.
Most of its constituents seep into the surrounding groundwater. From there it eventually reaches agricultural areas, freshwater rivers and lakes, and freshwater sources.
In the 1970s the US Air Force did some research on animals. It has been proven that this compound is toxic to the environment and health. However, we currently do not know of a better alternative for extinguishing liquid fires.
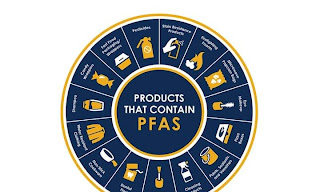
Frankly, the situation is worse. This fire extinguishing foam is one of thousands of man-made chemicals. Such substances are called “per- and polyfluoroalkyl substances” (PFAS). They are also known as “Forever Chemicals”. These plastics, capable of resisting water, heat and grease, were developed in the 1950s. Since then, these have been used for various purposes. Commonly used in kitchen utensils, cloth and paper products. They also cater to industries ranging from aerospace, semiconductors to automobiles.
Tiny particles of PFAS gradually accumulate in water. This is how they get into crops, fish and other meaty foods. The range of these particles is so wide that they can be hidden in the dust of bookshelves at home, entering our bodies through breathing. Research has shown that almost everyone on Earth today may have two types of PFAS in their blood: perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA).
It seems that there is no escape from these. Let’s know, what is this ‘Forever Chemical’ or ‘Eternal Chemical Substance’?
What Is Forever Chemical?
Most PFAS chemicals are now known as persistent chemicals. Because they cannot be broken. Long-term exposure to PFASs, or if they enter the body, can cause a variety of problems. Such as reduced fertility, hormonal changes, increased cholesterol, weakened immune system, risk of cancer, various delays in growth and learning in children, etc.
Efforts to eliminate PFAS from the environment have increased over the past few years. Researchers are working on some alternatives to the methods used so far. For example, they are considering incinerating PFAS compounds.
In fact, breaking down these compounds requires a lot of heat (about 400 degrees Celsius) and pressure. This causes air pollution again. These PFAS compounds can be compared to the Eternal Ring in Tolkien’s book ‘The Lord of the Rings’. There one character solemnly tells another, “… any force is useless to break this ring. Even if you hit it with a heavy sledge-hammer it will not corrode … no blacksmith has the tools to melt it … not even a furnace will work. … The only way: to find the cracks.”
Over the past decade, many scientists have been researching the molecules of PFAS. They are looking for an alternative method to break the anus. So far, fire cannot burn it to ashes; Other chemicals also cannot break down PFAS; Even bacteria that can ingest certain synthetic compounds and destroy them fail to work in the case of PFAS.
Northwestern University professor William Dichtel is a chemistry researcher. His work aims to find a practical way to destroy PFAS. PFAS can be found in literally hundreds of thousands of products today. The concern is that consumer-facing products should not contain such toxic ingredients.
Professor William Dichtel and his team have developed a promising technique. In August 2022, they published a research paper on this topic in the journal Science. It has been shown that at least ten PFAS compounds can be destroyed by a combination of low temperatures and relatively inexpensive, simple reagents.
Breaking Strong Bonds Of PFAS
PFAS molecules are made up of a long chain of carbon and fluorine atoms. These carbon and fluorine molecules are strongly bonded to each other. Professor Dichtel experimented by adding charged oxygen atoms to one end of this chain. Dichtel’s team discovered that oxygen atoms weaken PFAS.
They added a solvent called dimethyl sulfoxide to the PFAS. This substance can heat the PFAS molecules to between 80 and 120 degrees Celsius. Another substance is sodium hydroxide. It is a common reagent. The element or compound used to cause a chemical reaction is called a reactant. Reagents participate in chemical reactions and form new substances. Sodium hydroxide aids in the chemical reaction between PFAS. So that was also part of the solution.
This magical combination forces charged oxygen atoms to escape. and also dissociates fluorine atoms from PFAS atoms. And the new substance fluorine is created, which dentists use to strengthen the enamel of children’s teeth.
It takes 12 hours to complete the entire process. During this period, research found that more than 90 percent of the chemicals in PFAS could be converted into safer carbon products. High-risk operations such as water treatment plants will be the main target of Dichtel’s proposed solutions.
There is also the possibility of destroying atoms mechanically. Dichtel’s team published another paper on a technique to use high mechanical forces to break apart these atoms. This technology can be scaled down, thereby purifying any beverage.
Another technique called ‘supercritical water oxidation’ is also promising. A few nonprofit companies use this technology to clean water contaminated with PFAS. In this, water is heated to 374 degrees Celsius and pressurized to 22 MPa As a result, the PFAS molecule breaks into smaller pieces. Which includes hydrofluoric acid. Sodium hydroxide neutralizes the acid. This results in non-toxic salt and PFAS-free water.
Regulations On Keeping The Environment Free Of PFASs Lag Far Behind reality
About 12,000 different PFAS compounds currently exist in the environment. Most of them are harmful to humans and the environment. Since they do not decay, they are constantly increasing in the environment. Only two types of PFAS, polyvinyl fluoride and polytetrafluoroethylene, eventually break down.
At least two companies in the U.S. have begun removing known toxic PFAS compounds from their products under government orders. Yet in many cases these companies simply replace the banned compound with another PFAS compound. Considering plastics, PFAS-E is the villain that keeps coming back again and again, refusing to be destroyed in any way.
To protect the environment and life from PFAS, these PFAS compounds must be treated as a single class of chemicals as a whole, not individually, as is currently done.
Because, if each compound is studied separately, it will take a long time. And the continued use of thousands of other PFAS compounds will be ignored.
Removing PFAS from water is only part of the problem. This is also an important part of the problem. But, it is already clear that this is not going to be an easy and seemingly magical solution.
The ultimate solution is to dramatically reduce the use of PFAS. Its use beyond essential social needs should be strictly limited. For example, it can be used in low-rate semiconductor production. Currently, many scientists believe that the use of PFAS should be limited.
Scientists say PFAS should be removed from all consumer-facing products. PFAS must be destroyed wherever it exists. In most cases its use should be discontinued. Then gradually the problem will be solved.